Octahedrite
| Octahedrite | |
|---|---|
| — Structural class — | |
 Octahedrite from Toluca | |
| Compositional type | Iron |
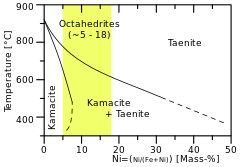 A phase diagram showing the link between structural and chemical classification. | |
Octahedrites are the most common structural class of iron meteorites. The structures occur because the meteoric iron has a certain nickel concentration that leads to the exsolution of kamacite out of taenite while cooling.
Structure
[edit]Octahedrites derive their name from the crystal structure paralleling an octahedron. Opposite faces are parallel so, although an octahedron has 8 faces, there are only 4 sets of kamacite plates.
Due to a long cooling time in the interior of the parent asteroids, these alloys have crystallized into intermixed millimeter-sized bands (from about 0.2 mm to 5 cm).[1] When polished and acid etched the classic Widmanstätten patterns of intersecting lines of lamellar kamacite, are visible.
In gaps between the kamacite and taenite lamellae, a fine-grained mixture called plessite is often found. An iron nickel phosphide, schreibersite, is present in most nickel-iron meteorites, as well as an iron-nickel-cobalt carbide, cohenite. Graphite and troilite occur in rounded nodules up to several cm in size.[2]
Subgroups
[edit]
Octahedrites can be grouped by the dimensions of kamacite lamellae in the Widmanstätten pattern, which are related to the nickel content:[3]
- Coarsest octahedrites, lamellae width >3.3 mm, 5-9% Ni, symbol Ogg
- Coarse octahedrites, lamellae 1.3-3.3 mm, 6.5-8.5% Ni, symbol Og
- Medium octahedrites, lamellae 0.5-1.3 mm, 7-13% Ni, symbol Om
- Fine octahedrites, lamellae 0.2-0.5 mm, 7.5-13% Ni, symbol Of
- Finest octahedrites, lamellae <0.2 mm, 17-18% Ni, symbol Off
- Plessitic octahedrites, kamacite spindles, a transitional structure between octahedrites and ataxites,[4] 9-18% Ni, symbol Opl
Mineral
[edit]Octahedrite is an obsolete synonym for anatase, one of the three known titanium dioxide minerals.[citation needed]
See also
[edit]References
[edit]- ^ Goldstein, J.I; Scott, E.R.D; Chabot, N.L (2009). "Iron meteorites: Crystallization, thermal history, parent bodies, and origin". Chemie der Erde – Geochemistry. 69 (4): 293–325. Bibcode:2009ChEG...69..293G. doi:10.1016/j.chemer.2009.01.002.
- ^ Vagn F. Buchwald: Handbook of Iron Meteorites. University of California Press, 1975.
- ^ James H. Shirley,Rhodes Whitmore Fairbridge, Encyclopedia of planetary sciences, Springer, 1997. ISBN 978-0-412-06951-2
- ^ Geochimica et Cosmochimica Acta, Volume 45, Ed. 9-12
